Altered redox signaling and oxidative stress: the emerging faces of CCM disease
Novel experimental evidence suggests that altered redox signalling and oxidative stress might play a role in the pathogenesis of Cerebral Cavernous Malformations (CCM) (Goitre et al., 2014).
Loss-of-function mutations of CCM genes, including the KRIT1 gene (CCM1), have been clearly associated with CCM pathogenesis. However, experiments in conditional knockout mouse models have demonstrated that the homozygous loss of CCM genes is not sufficient to induce CCM lesions, suggesting that additional factors, possibly specific for the neurovascular microenvironment, are necessary to cause CCM disease (Boulday et al., 2011).
Indeed, it has been previously reported that KRIT1 is involved in the maintenance of the intracellular reactive oxygen species (ROS) homeostasis to prevent ROS-induced cellular dysfunctions, including a reduced ability to maintain a quiescent state, suggesting that locally increased ROS and oxidative stress may act as additive susceptibility factors in the initiation and progression of CCM disease (Goitre et al., 2010).
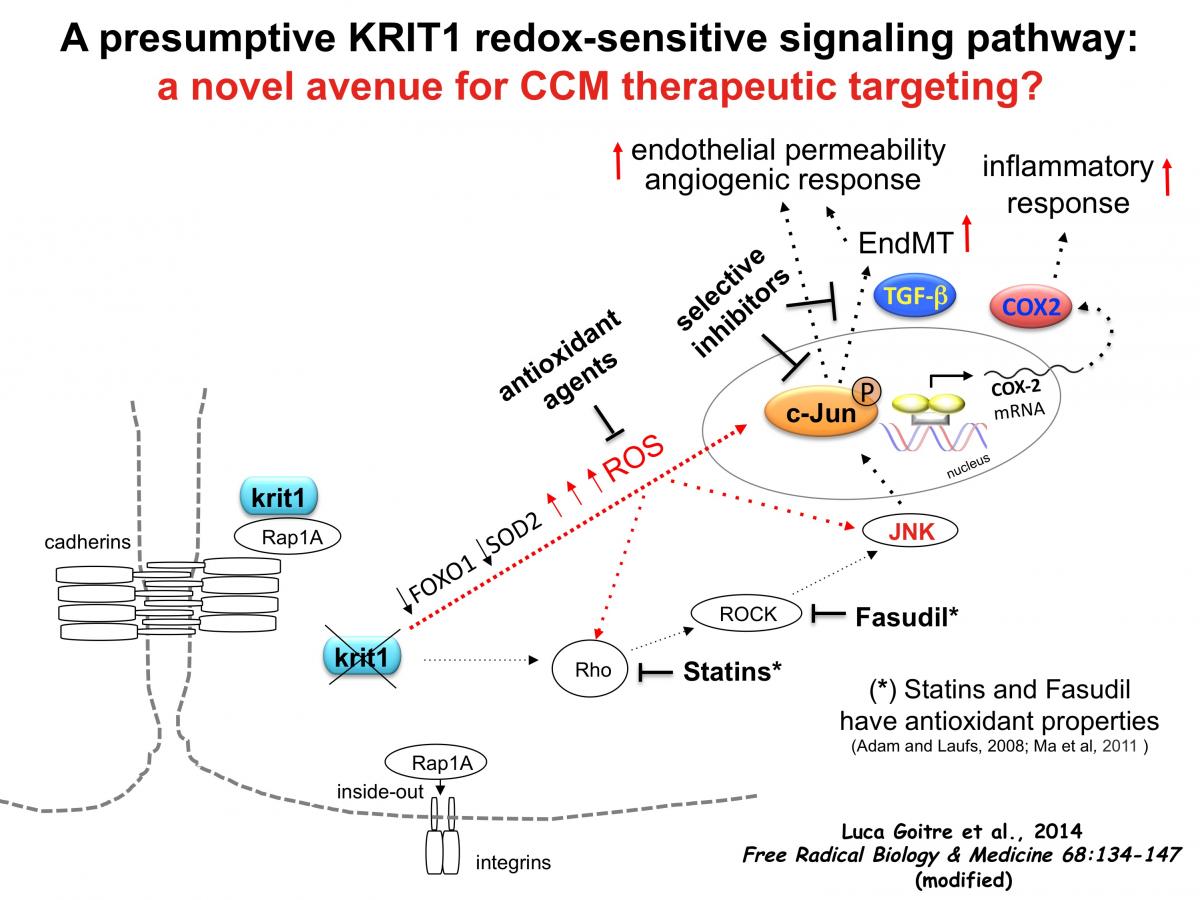
Accordingly, the recent paper by Luca Goitre et al., published in Free Radical Biology and Medicine(March 2014, Vol. 68, pp. 134-147), now shows that KRIT1 loss-of-function leads to enhanced expression and phosphorylation of the redox-sensitive protein c-Jun, a prominent member of the AP-1 family of transcription factors, as well as induction of its downstream target COX-2, both in cellular models and human CCM tissues. Furthermore, it demonstrates that c-Jun up-regulation can be reversed by either KRIT1 re-expression or ROS scavenging, whereas KRIT1 over-expression prevents forced up-regulation of c-Jun induced by oxidative stimuli. Taken together with the reported role of c-Jun in vascular dysfunctions triggered by oxidative stress, these novel findings suggest an important role for ROS and the redox-sensitive transcription factor c-Jun in CCM pathogenesis, pointing to a model mechanism whereby the dose of KRIT1 may be relevant in preventing c-Jun-dependent induction of vascular dysfunctions triggered by oxidative stress (Graphical model).
Consistently, c-Jun up-regulation has been linked with pathological angiogenesis and microvascular diseases in humans, whereas its inhibition has been shown to be effective as a rescue therapy for these diseases (Folkman, 2004; Zhang et al., 2004; Fahmy et al., 2006; Zhang et al., 2006).
Moreover, the recently reported findings related to a role of TGF-β in CCM patients (Maddaluno et al., 2013) might also be connected to and downstream of deregulation of oxidative stress and c-Jun activity. Indeed, whereas there is clear evidence that ROS can stimulate the activation of the TGF-β pathway with important consequences on cellular functions (Fukawa et al., 2012; Gonzalez-Ramos et al., 2012; Rhyu et al., 2005; Gorowiec et al., 2012), it has been demonstrated that this ROS-mediated regulation is dependent on AP-1 transcriptional activity (Gonzalez-Ramos et al., 2012).
Importantly, the recent findings by Goitre et al. (Free Radic Biol Med. 2014 Mar;68:134-147) provide also an alternative explanation to the suggested effectiveness of fasudil and statins as potential therapy for CCM disease (Whitehead et al., 2009; Li and Whitehead, 2010; McDonald et al., 2012). Indeed, whereas it has been demonstrated that the Rho GTPase pathway can be directly activated by ROS (Aghajanian et al., 2009), there is clear evidence that both fasudil and statins exert powerful intracellular antioxidant activities in endothelial cells, including the inhibition of superoxide production and the improvement of both ROS scavenging and NO bioavailability (Adam and Laufs, 2008; Kuhlmann et al., 2008; Ma et al., 2011).
Future progress toward this novel perspective should pave the way for the development of novel, safe and effective therapeutic strategies for prevention and treatment of CCM disease.
Reprint requests to Prof. Saverio Francesco Retta, Department of Clinical and Biological Sciences, University of Torino, Italy (e-mail: francesco.retta@unito.it).

